“I am Saruman the Wise, Saruman Ring-maker, Saruman of Many Colours!”
I looked then and saw that his robes, which had seemed white, were not so, but were woven of all colours, and if he moved they shimmered and changed hue so that the eye was bewildered.
“I liked white better,” I said.
“White!” he sneered. “It serves as a beginning. White cloth may be dyed. The white page can be overwritten; and the white light can be broken.”
“In which case it is no longer white,” said I. “And he that breaks a thing to find out what it is has left the path of wisdom.”
–Gandalf, recounting his confrontation with Saruman in The Fellowship of the Ring
Even as a kid, reading J. R. R. Tolkien’s The Lord of the Rings at the golden age of twelve or so, Gandalf’s response to Saruman never sat well with me. Splitting white light into its component colors is awesome, and taking things apart is the best way to learn how they work. Knowing how things work is the first step toward making them work better, a process that leads to the technologies that make modern life comfortable enough to, among other things, provide Oxford dons with enough free time to construct elaborate fantasy universes.
With an attitude like that, it was probably inevitable that I would grow up to be a scientist. And as I grew up to become a physicist working with atoms and lasers, I’ve only become more convinced that Gandalf is wrong. Splitting light isn’t a mistake, it’s the first step on the path toward our modern understanding of the universe.
Splitting Light and the Birth of Quantum Physics
The science of splitting light into its component colors is called spectroscopy, which began in earnest in the mid-1800’s with the discovery that different chemical elements emitted different colors of light. The best-known examples are the characteristic red-orange glow of neon lights and the yellow-orange of sodium vapor streetlights, but every element emits its own unique set of wavelengths of light. These characteristic colors are called “spectral lines” because they usually appear as bright stripes in the spread-out spectrum of light from some source. They can be used to identify the composition of hot objects, and even discover new elements: in 1868 helium was first detected as an unexplained line in the spectrum of the Sun.
These spectral lines are undeniably useful, but scientists did not at first understand why atoms emit some wavelengths but not others. This problem was one of the great mysteries facing physics in the late 1800’s. An essential clue to the origin of spectral lines was provided by German schoolteacher Johann Balmer in 1885, who found a simple mathematical formula that described the wavelengths of the lines in hydrogen’s exceptionally simple visible spectrum. Johannes Rydberg expanded Balmer’s formula to encompass the ultraviolet and infrared lines in hydrogen just a few years later. The physics underlying the formulae, though, remained mysterious for the next three decades.
The first successful model of the physics underlying the Rydberg formula came from the Danish physicist Niels Bohr in 1913. Bohr’s model of hydrogen builds on a picture of the atom introduced by Ernest Rutherford in 1911, which is the progenitor of the cartoon atom everybody learns about in elementary school, with electrons orbiting a positively charged nucleus. Rutherford’s model had a major flaw, however: according to the known physics of electricity and magnetism, an orbiting electron should spray radiation outward in all directions, at a wide range of wavelengths, thereby losing energy, and spiraling inward to crash into the nucleus. Classical physics does not allow stable solar-system-like atoms, or allow them to produce light at well-defined frequencies.
 In order to match the Rydberg formula, Bohr made a radical leap: he proposed that, in defiance of everything known about classical physics, an electron circling the nucleus of an atom in certain special orbits would not emit any light. In Bohr’s model, atoms emit light only when they move between these “allowed states,” and the color of the emitted light depends on the difference between the energies of the initial and final states.
In order to match the Rydberg formula, Bohr made a radical leap: he proposed that, in defiance of everything known about classical physics, an electron circling the nucleus of an atom in certain special orbits would not emit any light. In Bohr’s model, atoms emit light only when they move between these “allowed states,” and the color of the emitted light depends on the difference between the energies of the initial and final states.
Bohr’s model successfully explains the spectrum of hydrogen, but his rule for determining the special allowed orbits was completely arbitrary and demanded a deeper explanation. In 1924, a French Ph.D. student named Louis de Broglie realized that he could justify Bohr’s model by saying that electrons have wave-like properties: Bohr’s special orbits were simply those whose circumference was an integer times the wavelength of an orbiting electron. De Broglie’s prediction was just as radical as Bohr’s – his professors had no idea what to make of it at first, and they were reluctant to accept it until Einstein proclaimed it brilliant. Shocking though it was, de Broglie’s idea of matter waves was confirmed experimentally a few years later when physicists directly observed electrons behaving like waves. As a result, the new science of quantum mechanics was launched.
The modern theory of quantum mechanics is far more complicated than the simple models of Bohr and de Broglie (and much stranger), but it works brilliantly, correctly predicting the wavelengths of light emitted by hydrogen to some 14 decimal places. Quantum physics underlies essentially all modern technology: we can make computer chips because we understand the quantum nature of electrons and can manipulate their behavior inside materials like silicon. We can make the lasers that are crucial to fiber-optic telecommunications because we understand the quantum nature of light, and its interaction with atoms. The modern internet and all its revolutionary effects would be impossible without quantum physics, and while you might question the amount of wisdom to be found on the internet, the path to it unquestionably begins with the splitting of light.
Splitting Light, Timekeeping, and Navigation
Quantum mechanics and precision spectroscopy also allow us to measure time to astonishing precision. When atoms emit light, the oscillation frequency of that light is determined by the energy separation between two allowed states in the atom. That difference is determined by quantum mechanics, and is the same for every atom of that element. The light’s oscillation can therefore be treated as the “ticking” for a very precise clock, with atoms serving as perfect reference sources to verify that the frequency is correct.
The modern definition of time is thus based on spectroscopy: one second is defined as 9,192,631,770 oscillations of the radiation emitted by cesium-133 atoms moving between two specific energy levels. Modern cesium atomic clocks can measure this to astonishing precision: the cesium fountain clock at the National Physical Laboratory in the U.K. uses spectroscopy to match the cesium frequency so precisely that it would take more than 130 million years to lose one second. And experimental clocks based on aluminum ions, at the National Institute of Standards and Technology in Boulder, Colorado, are even more accurate, taking a few billion years to lose one second.
Such fantastic timing accuracy allows physicists to directly measure the predictions of Einstein’s theory of relativity on human scales. Special relativity tells us that moving clocks “tick” at a rate that is slower than an identical stationary clock, while general relativity tells us that a clock at high altitude will tick faster than an identical clock at sea level. These predictions have been verified by atomic clocks in jet planes, but the aluminum-ion clocks are so precise they can see a moving clock run slow at speeds as low as 4 m/s (about 9mph), and see a higher clock run fast due to a change of just 33cm (about a foot).
Precision timekeeping is also essential for modern navigation. The Global Positioning System (GPS) is a network of cesium atomic clocks in satellites orbiting the Earth. Each satellite broadcasts the time according to its clock, and a GPS receiver in your car or cell phone picks up radio signals from several satellites. Measuring the difference between the arrival times for signals from different satellites allows a computer to calculate the distance from each satellite to your receiver; knowing the distance to three satellites specifies your position on the surface of the Earth to within a few meters. GPS may not be necessary to walk the path of wisdom, but it can be essential for keeping you on the path to home, and it all begins with the splitting of light.
Splitting Light and the Fate of the Universe
Finally, separating light into different colors is also the first step toward our modern understanding of the origin, history, and eventual fate of the universe. Not only does the light emitted by distant stars tell us their composition, through the spectral lines emitted by the different elements, it also tells us their velocity through the Doppler effect. This is a shift in the frequency of waves emitted by a moving source, and the most familiar example is the characteristic eeeeeee-ooowwwww sound of a fast moving car going by. As the car approaches, the sound waves from its engine Doppler shift up in pitch (higher frequencies, shorter wavelengths), and as it recedes, they Doppler shift down in pitch (lower frequencies, longer wavelengths).
The same shift takes place with light: light from approaching objects shifts toward the blue end of the visible spectrum, and light from receding objects shifts toward the red. The larger the shift, the faster the object is moving: therefore, astronomers can tell how fast and which way a distant star is moving by comparing its spectral lines to the same lines from a source on Earth.
In the late 1920’s, the American astronomer Edwin Hubble measured the spectrum of light emitted by 46 different galaxies. Nearly all of them showed spectral lines shifted to the red, indicating that they were moving away from us. Furthermore, the more distant galaxies had larger shifts, indicating that they were moving away faster. The galaxies’ speed was proportional to distance, so a galaxy that was twice as distant was moving twice as fast. This relationship, now known as “Hubble’s Law,” has been confirmed by numerous other observations.
Hubble’s result, unexpected at the time, is explained very naturally by a model in which the universe is expanding, now known as the “Big Bang” model (a name given in scorn but adopted with pride). According to our best understanding, the universe began as a single, very hot, extremely dense point around 13.7 billion years ago, and has been expanding and cooling ever since. Further support for this model was again provided by measuring the colors of light, this time the “cosmic microwave background” radiation left over from a time about 300,000 years after the Big Bang. In the 1940’s, Ralph Alpher and Robert Herman predicted that this leftover radiation would have the same distribution of wavelengths as the spectrum of light emitted by an object at 5 kelvin (five degrees above absolute zero). When this background radiation was detected by Arno Penzias and Robert Wilson in 1965, its temperature was 2.7 K. The cosmic microwave background is one of the most important bits of evidence for the Big Bang, and measuring the subtle variations in its spectrum provides our very best information about the conditions of the early universe.
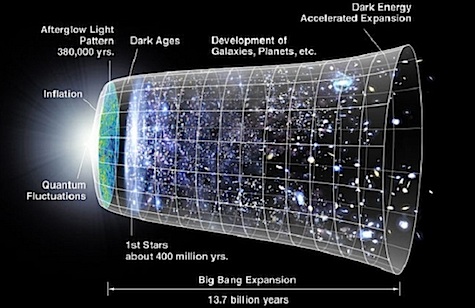
Spectroscopy also allows us to determine the eventual fate of the universe. In the late 1990’s, astronomers extended Hubble’s law to vastly greater distances by using supernovae to accurately determine the distance to galaxies formed only a few billion years after the Big Bang. They expected the spectra of these galaxies to show that the expansion was slowing down over time, due to the force of gravity pulling galaxies back together. Instead they found the opposite: the expansion of the universe is accelerating. A mysterious substance known as “dark energy” is pushing the universe outwards, causing it to expand faster and faster as time goes on. The expansion will continue forever, with the universe becoming infinitely large and increasingly empty. The 2011 Nobel Prize in Physics was awarded to Saul Perlmutter, Brian Schmidt, and Adam Riess for the discovery of the accelerating expansion.
Numerous questions remain to be answered—what is the exact nature of the dark energy? what caused the Big Bang?—but the first step on the path to understanding where we came from and where we’re going involves the splitting of light.
Far from being a step off the path of wisdom, then, the splitting of light is the essential first step toward modern physics. While this might not have held much appeal for Gandalf or Tolkien (who had some Luddite tendencies), those of us who enjoy the internet, GPS, and other benefits of modern science have numerous reasons to be grateful for spectroscopy. In this one thing (but probably only this one thing), we ought to be on Saruman’s side.
Chad Orzel is a physics professor at Union College in Schenectady, NY, and the author of two popular science books, How to Teach Physics to Your Dog and How to Teach Relativity to Your Dog, that explain modern physics through conversations with his dog, Emmy. He runs the physics blog Uncertain Principles at the ScienceBlogs blog network.